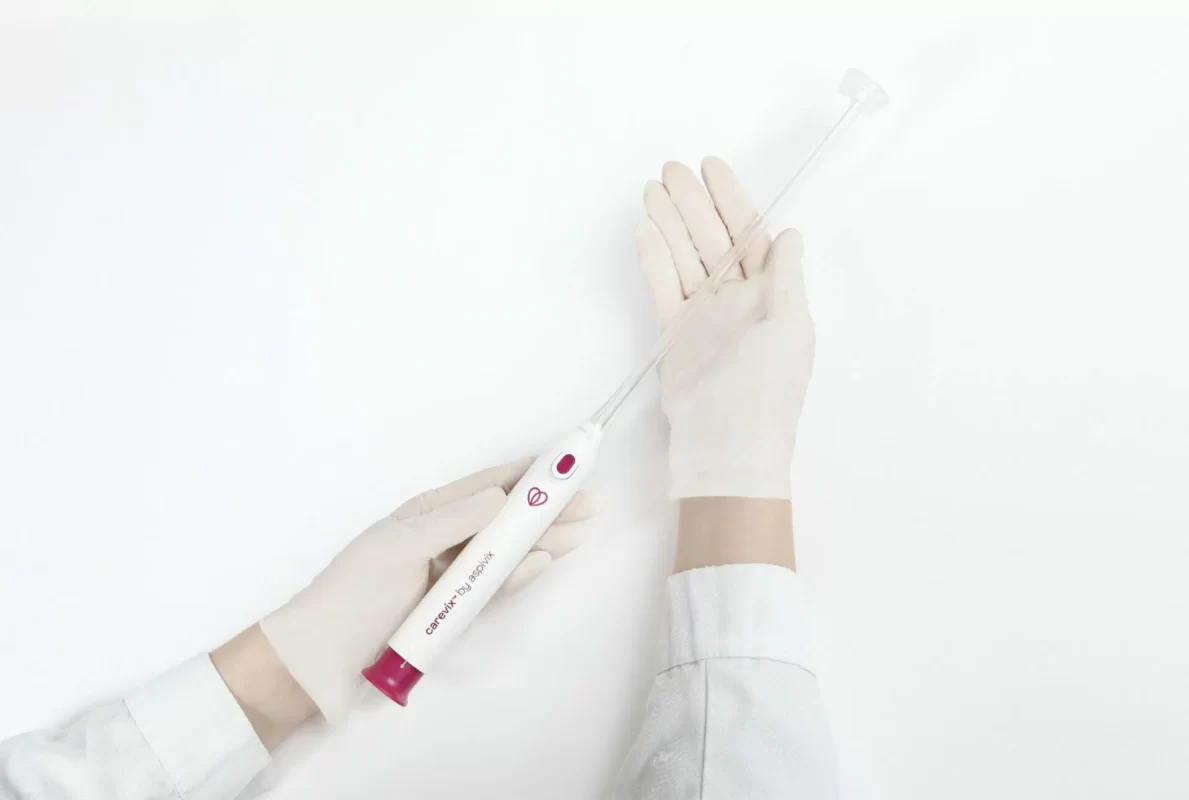
HEMEX is proud to announce that our portfolio company ASPIVIX has received 510(k) clearance of Carevix™ from the U.S. Food and Drug Administration (FDA). ASPIVIX’s novel Cervical Stabilizer will allow millions of women across the USA access to significantly less painful treatments and IUD insertions.
ASPIVIX 510(k) Clearance by FDA news release_Draft_EN-V3
About HEMEX

HEMEX is a CRO providing clinical services to European biotech and medtech companies, assisting them throughout the clinical development process to ensure that products reach the market swiftly. Located in the Basel region, a major life sciences hub, and with offices in Germany and Benelux, we are well positioned to serve small and medium enterprises (SMEs) with multinational clinical projects. The comprehensive package of services we provide includes clinical operations, quality management, regulatory guidance, grant writing services, and fundraising options. At HEMEX, we guarantee fast and clear communication, local accessibility, and transparency to meet the objectives of our clients. For more information, visit https://hemex.ch/