Resistell recruits first patients for international clinical study
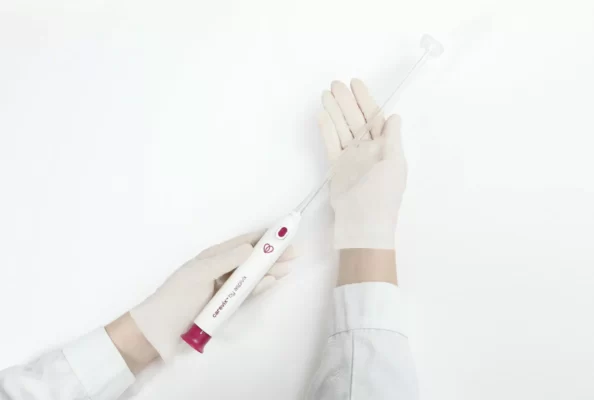
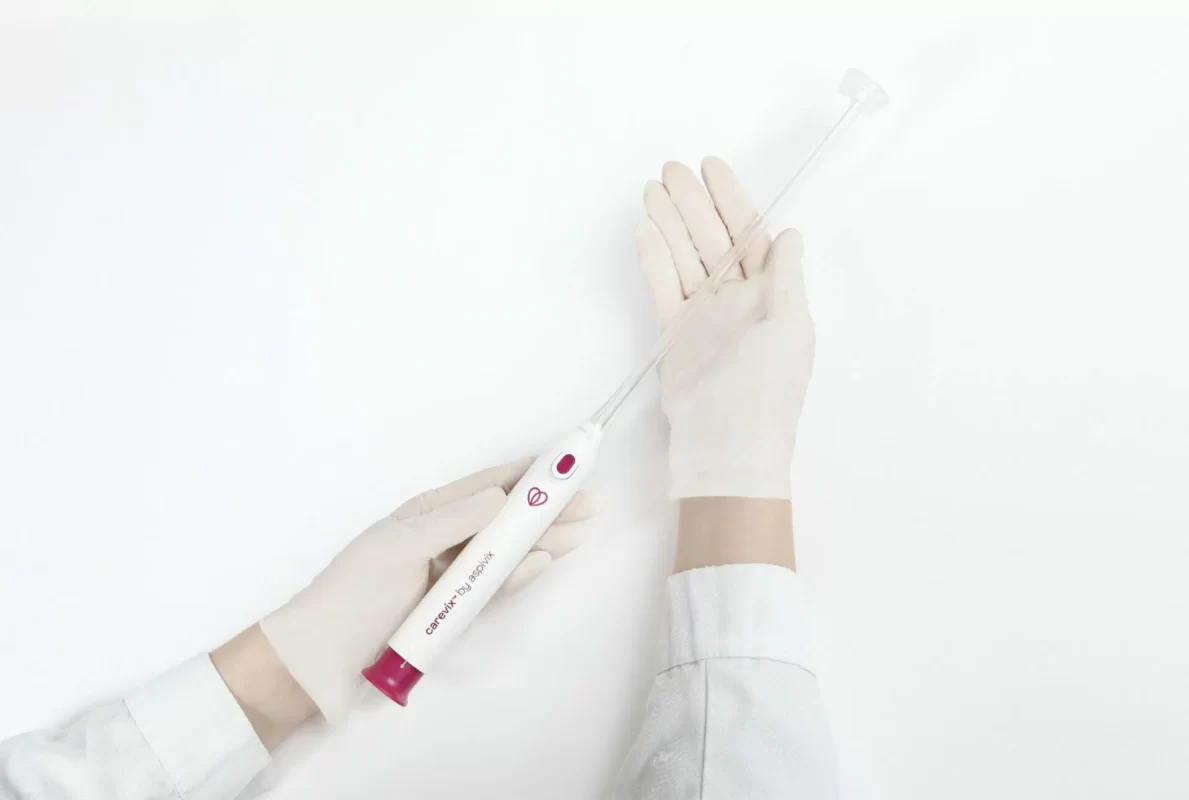
HEMEX is proud to announce that our portfolio company ASPIVIX has received 510(k) clearance of Carevix™ from the U.S. Food and Drug Administration (FDA). ASPIVIX’s novel Cervical Stabilizer will allow millions of women across the USA access to significantly less painful treatments and IUD insertions.
We are thrilled to share that HEMEX is part of the investment consortium financing the series B1 of hemotune AG. At HEMEX, we look forward to support hemotune on the journey to making the HemoSystem available to patients globally.
This milestone shows the devotion of our QA team to ensuring HEMEX’s high standards and commitment to quality. Achieving this certification was one of the milestones we wished to accomplish in 2022.
We are thrilled to share that HEMEX is part of the investment consortium financing the series B1 of hemotune AG. At HEMEX, we look forward to support hemotune on the journey to making the HemoSystem available to patients globally.
We’re delighted to announce that our portfolio startup Resistell AG has completed the pilot phase of its clinical Performance Evaluation
Study (PES) with 100% accuracy and at least 7 hours shorter time-to-result (TTR) compared to gold standard methods.