New Year’s resolutions can be a powerful way to make positive changes in life and achieve goals.
Resistell recruits first patients for international clinical study
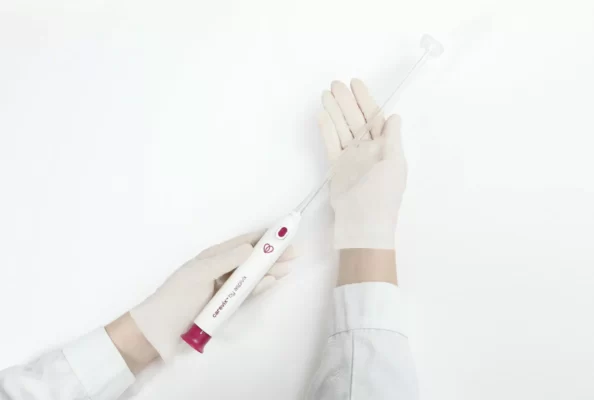
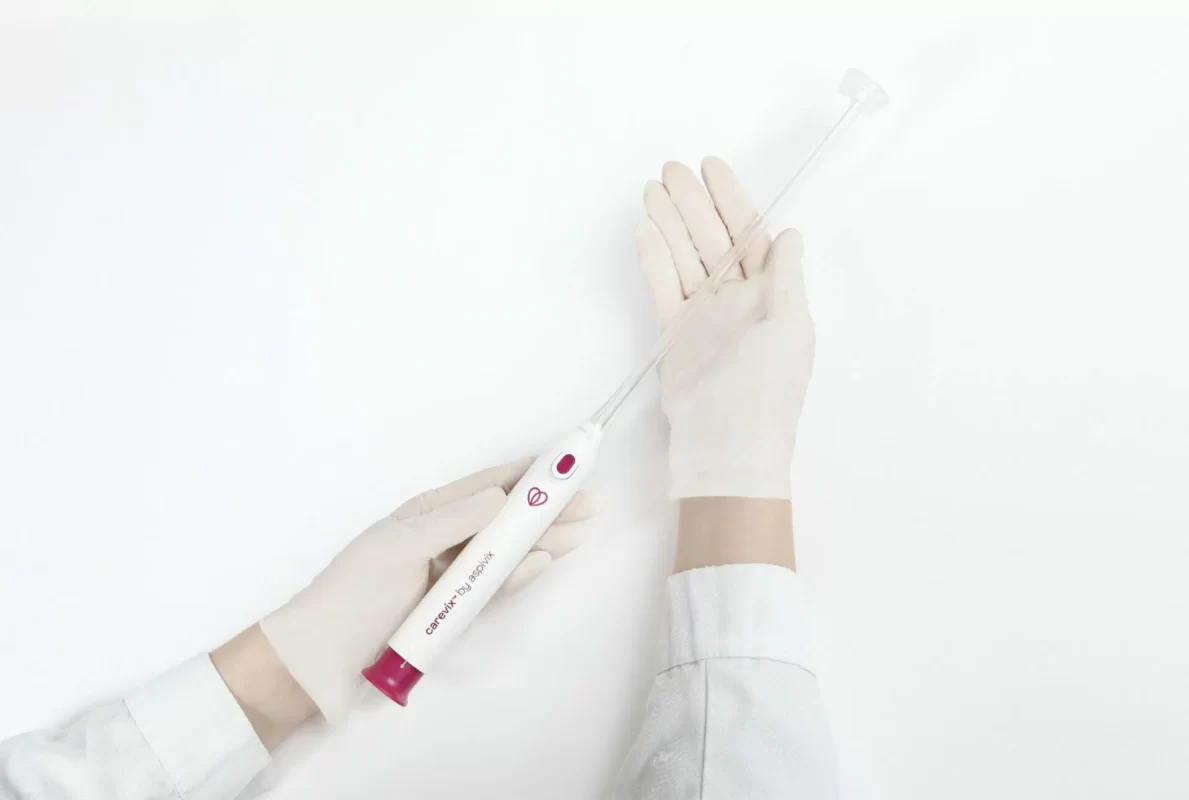
HEMEX is proud to announce that our portfolio company ASPIVIX has received 510(k) clearance of Carevix™ from the U.S. Food and Drug Administration (FDA). ASPIVIX’s novel Cervical Stabilizer will allow millions of women across the USA access to significantly less painful treatments and IUD insertions.
This milestone shows the devotion of our QA team to ensuring HEMEX’s high standards and commitment to quality. Achieving this certification was one of the milestones we wished to accomplish in 2022.
We are thrilled to share that HEMEX is part of the investment consortium financing the series B1 of hemotune AG. At HEMEX, we look forward to support hemotune on the journey to making the HemoSystem available to patients globally.
Today, on the fourth of February 2022, we want to spread awareness about the gap in cancer care and support the initiative from the Union for International Cancer Control (UICC), which strives to close the cancer care gap.
We’re delighted to announce that our portfolio startup Resistell AG has completed the pilot phase of its clinical Performance Evaluation
Study (PES) with 100% accuracy and at least 7 hours shorter time-to-result (TTR) compared to gold standard methods.
Resistell has started the Performance Evaluation Study (PES) of its groundbreaking nanomotion technology at Lausanne University Hospital. At HEMEX, are very happy to be part of this project, as we will be monitoring the clinical trial over the next 12 months.
On HEMEX’s Investment Showcase today we tackled the pain, bleeding, and tissue tear that women worldwide experience when undergoing common gynaecological procedures. Our portfolio company ASPIVIX is developing the next-generation suction-based cervical device that will improve these traumatic experiences.
Our portfolio company Opterion Health AG announced today the successful agreement of a EUR 8.4m Bridge Financing
Round. The proceeds from this financing round will be used to complete preclinical activities and prepare for entry into Phase I. Heartfelt congratulations on your success, Opterion!