Resistell recruits first patients for international clinical study
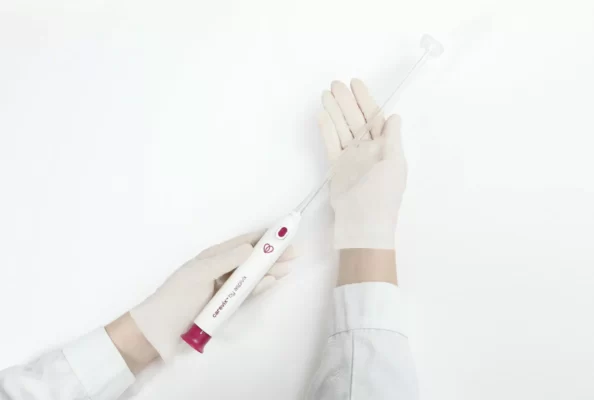
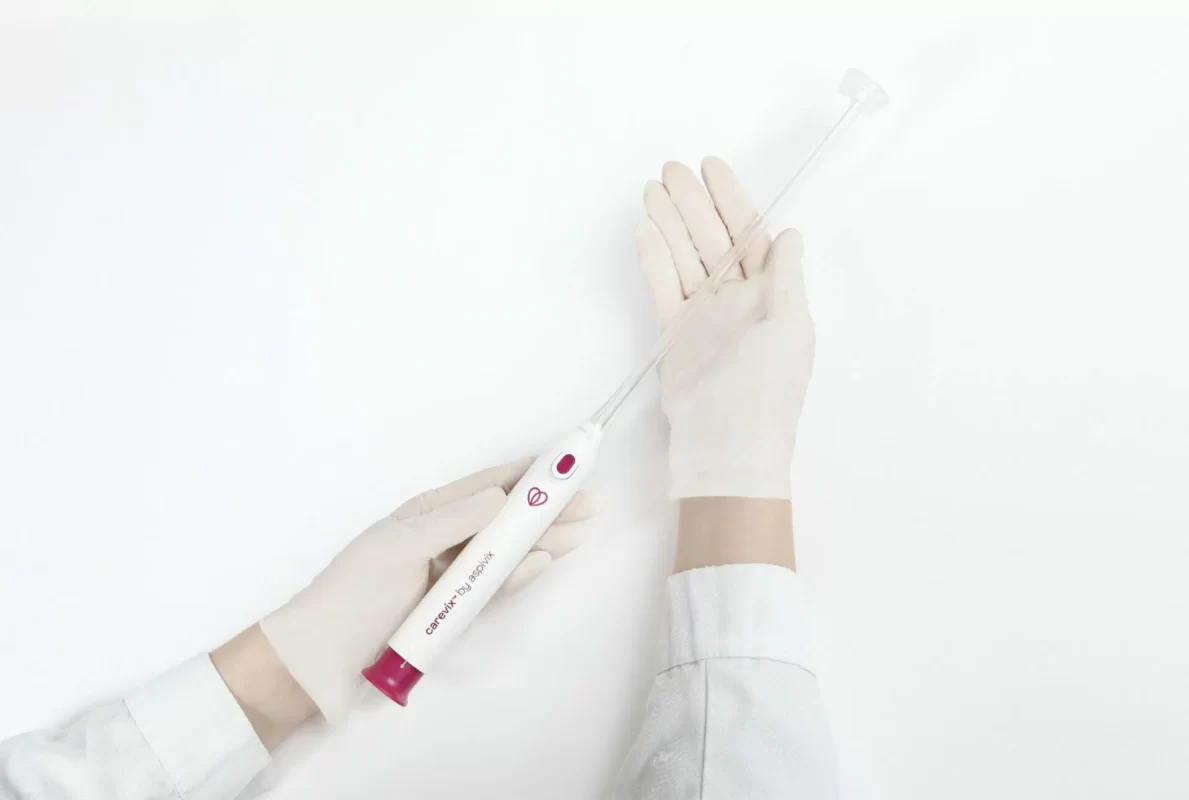
HEMEX is proud to announce that our portfolio company ASPIVIX has received 510(k) clearance of Carevix™ from the U.S. Food and Drug Administration (FDA). ASPIVIX’s novel Cervical Stabilizer will allow millions of women across the USA access to significantly less painful treatments and IUD insertions.
We are thrilled to share that HEMEX is part of the investment consortium financing the series B1 of hemotune AG. At HEMEX, we look forward to support hemotune on the journey to making the HemoSystem available to patients globally.
Today, on the fourth of February 2022, we want to spread awareness about the gap in cancer care and support the initiative from the Union for International Cancer Control (UICC), which strives to close the cancer care gap.
On HEMEX’s Investment Showcase today we tackled the pain, bleeding, and tissue tear that women worldwide experience when undergoing common gynaecological procedures. Our portfolio company ASPIVIX is developing the next-generation suction-based cervical device that will improve these traumatic experiences.
The New Year is an exciting time as we all focus on goals and resolutions. At HEMEX, we are focused on bringing you solutions at each critical step of the clinical development of your product. Together we can generate the accurate evidence needed for success!
In times of high uncertainty like this 2020 year, it is necessary to celebrate and savor the victories. We want to congratulate our portfolio company Sleepiz for its well-deserved success in winning collaboration with one of the three Executive MBA cohorts at the beginning of 2021